Soil 101
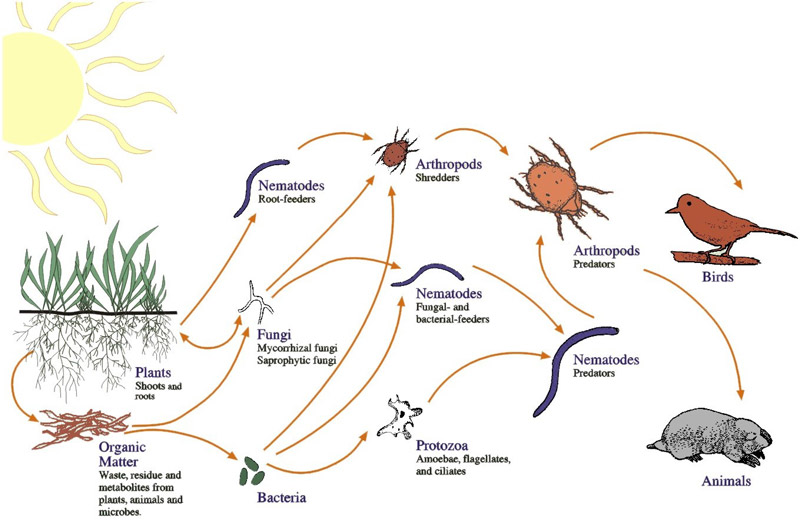
Soil is a complex habitat where plants and microorganisms live together.
Healthy, living soil is alive with a diverse population of microorganisms including bacteria, fungi, algae, protozoa, and nematodes. These organisms create a web of activity that decompose organic matter in soil to ensure that nutrients are usable by plants and crops. This results in continuous soil development, maintenance of soil structure, nutrient cycling, and various beneficial interactions with plant roots.
Understanding Soil
Soil is complex and is comprised of three basic components – a Biological, Chemical and Physical component. It is not simply dirt that we drag in doors on the bottom of our boot. Soil is a “living system” full of micro and macro organisms that contribute to agricultural productivity, soil and plant health as well as air and water quality. These soil organisms help to unlock nutrients in soil, fertilizers and organic amendment which then are made readily available to plant roots and tissues. Through specialized enzymes bacteria and fungi convert or make soluble sources of Nitrogen for example Ammonia Nitrogen more available to plant roots in a form such as Nitrate Nitrogen. In many cases Phosphorus is bound up tightly to Calcium in a compound found in many soils as Calcium Triphosphate. Bacteria and some fungi that contain special enzymes will break the bond between Calcium and phosphate allowing phosphorus to now be available to plant roots. Thus the biological component of soil mediates or influences chemical interactions in soil.
The chemical component of soil is best thought of as the Cation Exchange Capacity (CEC) of soil. The CEC of soil is a measure of its ability to hold and release various plant nutrients specifically positively charged nutrients. Something that has a positive (+) charge is called a cation and if it has a negative charge (-) it is called and anion. The word ion is simply a charged particle and a positive charge is attracted to a negative charge.
There are two types of Cations acidic or acid – forming cations and basic or alkaline – forming cations. The Hydrogen cation H+ and the Aluminum cation Al+++ are acid forming. A soil with high levels of H+ or Al+++ is an acid soil or a soil with a low pH. Positive charged cations such as Calcium, Magnesium, Potassium and Sodium are alkaline cations or basic cations and they are adsorbed onto a clay particle or Soil Organic Matter (SOM). A clay particle has a negative charge and attract positively charged nutrients while SOM contains both positive and negative charges and thus can adsorb or hold onto either cations or anions. Thus, the Cation Exchange Capacity is the measure of how many negatively charged sites are available in your soil. Soils high in organic matter may have a high CEC and thus can hold onto more or has lots of sites hold onto nutrients or cations while a sandy soil has a low CEC. Soils with some clay and organic matter may have CEC somewhere in between.
While soil has these exchanges sites the exposure or actual availability or access to these sites is related to the physical structure of soil. These sites can be on the surface of clay platelets or within soil pores. A soil’s Bulk Density is the amount of pore spaces (large or small) and the percentage of total of total volume of soil pores that is filled with water after irrigation and drainage is the soil’s Water Holding Capacity. When soil is saturated and allowed to drain the air replaces the volume of water drained. This is called Air Porosity. Having the right Water Holding Capacity, Bulk Density and Air Porosity can help to anchor plant roots, provide adequate water, air and nutrients to microorganisms which in turn provide plant needed nutrients to roots. Adequate access to exchanges sites will also contribute to better retention of nutrients for both microbes and plant roots.
Finally, in order to understand soil one cannot forget the role of plant roots or more specifically the microbe and plant interactions in soil. Plant roots and soil microorganisms exude or give off Hydrogen ions, H+ ions, and if enough of these H+ ions are given off then some of them surround the nutrient cation and get closer to the negatively (-) charged exchange site than the nutrient is, the H+ ions will fill the exchange site, neutralize the charge and the nutrient cation will be free of its static bond and can then be taken up by the plant or microorganism. Specifically, plant roots expire or breathe out carbon dioxide into the soil. This carbon dioxide (CO2) combines with water in the soil and forms carbonic acid, and the H+ Hydrogen ions from the carbonic acid are what replaces the cation nutrient on the exchange site. The Calcium ion that is held to the exchange site has a double-positive charge, written Ca++. When enough H+ ions surrounds the Calcium ion some of them get closer to the exchange site than the Ca++ ion is then plant is free to take the Ca++ up as a nutrient. In some cases when lots of H+ ions are present soil pH may decrease resulting in an acidic soil. Still plant roots can also exude Hydroxyl anions (0H-) which can raise soil pH resulting in an alkaline soil.
All of these nutrients (cations and anions) contributed by CEC, soil microorganisms, plant exudates and retained by clay content, organic matter result in the soil’s Electrical Conductivity. These ions or nutrients are dissolved in soil water and carry an electrical charge and thus EC is a measure of how much nutrients are available to plant roots to absorb.
Photosynthesis
![]()
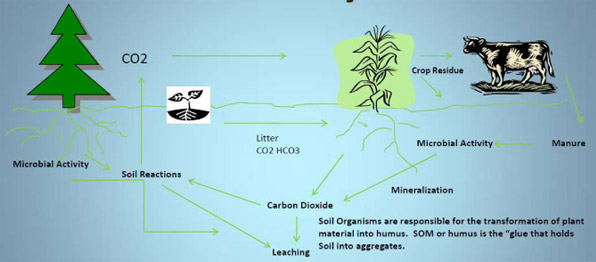
Through the process of photosynthesis, plants absorb CO2 from the atmosphere, transform it into plant carbon, sequester it in either above – or below-ground biomass and/or soil carbon.
There are two kinds of Soil Organic Matter:
- Short-term SOM is residue that is readily decomposed. Short-term SOM is a source of Nitrogen, Phosphorus, and Sulfur for plants.
- Long-term SOM (humus) is the carbon that resists decomposition and lasts a long time